Engine Oil Deep Dive - CAC's Comprehensive Look at Engine Oil and Flat-Tappet Camshaft Durability - Page 2 of 6
 |
 |
© 2009 by Hib Halverson
No use without permission, All Rights Reserved
![]() Discuss this article
Discuss this article
The second antiwear strategy is technology. Over time, better camshaft and lifter materials have been developed. This is not to say that every camshaft and lifter manufacturer uses those better materials, but they do exist and have been implemented by OE's and some aftermarket companies. Additionally, surface treatments, more robust and more permanent than phosphating, which dates to the mid-'50s, have been developed by the aftermarket, such as nitriding. Lastly, more stringent quality controls have, also, improved durability.
The third strategy is better lubrication. For over half a century, "extreme pressure" or "EP" lubricants have been added to engine oil to extend camshaft/lifter durability. They enhance the oil's ability to lubricate parts which rub against a small area of each other and are under high load while they rub. In the early-'50s, following introduction of higher valve spring pressures, there were problems with valvetrain wear. In response, car companies introduced camshafts with a sacrificial phosphate coating (which enhanced break-in reliability) and lifters made of hardenable alloys of cast iron. They also pressured the oil industry to reformulate engine oils to improve cam and lifter durability. Its response was a significant increase of EP additives in oil blends.
Mechanical properties, materials and lubrication can, also, increase wear. If a lobe is not properly tapered or a lifter face is not manufactured with the proper convex profile or surface finish, rapid wear will occur. If the materials are substandard (poor quality iron) or the surface is not treated properly (poor quality phosphating of the cam) the cam and lifters will fail. If the engine oil lacks a proper EP additive package (and this can be either too little or, sometimes, too much), the valvetrain may suffer poor durability.
Why ZDP Gets so Much Attention
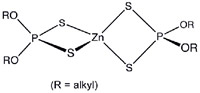
The most common EP additive in automotive engine oils is zinc dialkyldithio-phosphate (ZDDP), a family of coordination compounds of zinc and dithiophosphoric acid which, in longer chain, molecular derivatives, easily dissolve in engine oils. Known more commonly as "zinc dithiophosphate" (ZDP), "zinc phosphate" or, quite incorrectly, just "zinc", this compound was initially added to oil in the 1940s as an anti-corrosive/antioxidant. Later it was discovered to be an excellent extreme pressure lubricant.
When subjected to heat present at the lobe/lifter interface, ZDP decomposes into alcohol, zinc, sulfur and phosphorous. The alcohol evaporates and the zinc mostly washes away, leaving sulfur and phosphorous to combine with iron molecules on the surface of the cam lobe to make iron sulfide and iron phosphate, the two compounds which perform EP lubrication.
"The 'dithio' in 'zinc dithiophosphate' means for every phosphorous there are two sulfur molecules," Red Line Synthetic Oil Corporation's Vice President and top petro-chemical engineer, Roy Howell, told the Corvette Action Center.
"Sulfur is probably more important than zinc and phosphorous.
"The (cam and lifter) wear surfaces are rich in iron and sulfur with a lesser amount of phosphorous. The ZDP decomposes into a soft, thin film of iron sulfide and iron phosphate which prevents iron adhesion, or welding. The zinc doesn't do much. If you look at photomicrographs of cams and lifters, there's hardly any zinc coating, but there's a lot of iron sulfide coating and some iron phosphate coating.
"With this process, you trade adhesive wear for chemical wear. If you didn't have these soft films, which prevent iron from touching iron if you didn't have something in the middle, then you'd get adhesive wear-welding and that iron-to-iron weld would pull 'chunks' out of the lobe and follower.
"What makes zinc dialkyldithiophosphate unique is its precise thermal decomposition temperature which can be manipulated by changing the composition of the organic (alkyl) group attached to the phosphorous.
"If it decomposes at too low a temperature, chemical wear would occur where it is not needed but, if it occurred at a higher temperature, then some adhesion or welding, would already be taking place.
"There are a lot of different sulfur compounds," Howell continued, "but this one has 'precision-controlled' decomposition. In many of the others, the sulfur and the phosphorous are much more loosely bonded. There's a bigger 'range'. It might partially decompose at a lower temperature and finish at a higher temperature or, maybe, decompose only at a higher temperature, however, with ZDP-boom!-, like at 400°F, it starts to thermally decompose then react (with the surface of the lobe and lifter) to form those almost monomolecular soft films. As the lobe rubs against the follower, that film will get rubbed off and in the next revolution, the same thing happens again."
ZDP is slowly depleted by decomposition and evaporation, so eventually EP lubrication becomes inadequate. This is one reason oils need to be changed periodically.
While some petrochemical engineers consider sulfur of primary importance and some consumers misunderstand zinc content as benchmarking EP additives in oil; in reality, it is the phosphorous component about which the oil industry is most concerned.
Oil blending data prior to the early-'90s is difficult to acquire, but our research revealed that mass-marketed engine oil typically used by consumers from the late-1950s to the mid-'70s had enough ZDP to result in around 800 parts per million phosphorous content or "phos" as some engineers say.
In the early-'70s, "finger followers" were introduced in some single overhead camshaft (SOHC) engines. They presented a durability problem, initially, thought to be caused by insufficient EP lubrication. In response, from the late-'70s to the mid-1980s, phosphorus in most oils climbed to about 1000 PPM. Ironically, as experience with finger follower engines grew, it was eventually determined that, due to the location of the valvetrain in the engine, blowby driven corrosion was affecting the durability problem more than insufficient EP lubrication.
In the late-80s/early-90s, the oil industry began to decrease phosphorous, back towards 800 PPM because: 1) extra ZDP for finger followers proved unnecessary and alternative methods improved materials, other additives were found to enhance durability, 2) OE's were converting to roller lifters which required less EP lubrication, 3) OEs wanted to improve the longevity of emissions controls and 4) historically, as far back as the mid-'50s, 800 PPM phosphorous provided good durability of flat tappet cams and lifters in production OHV engines.
 |
 |